The Overlying Most Important Principle in This Report
What diseases could you treat If you had a treatment that could propagate new blood flow, grow nerves, calm the autoimmune response, fight infection, regrow collagen, and enhance glandular function? The answer to that question gives you an idea about the possibilities with platelet-rich plasma (PRP). That idea (together with the current research and the experience of more than three thousand doctors over a decade) is the theme behind the strategies discussed in this report.
Thinking about how antibiotics work helps you consider what conditions may be helped by antibiotics; this consideration of which conditions my be helped and which may not be helped by antibiotics happens so automatically that we may not consciously acknowledge the process.
For example, we would not think about antibiotics for the primary treatment of uterine fibroids because fibroids are not primarily caused by infection; antibiotics only treat infection. But, with a new therapy that is not as well known or understood, we may not be clear about the mechanism of the treatment; therefore, there can be confusion regarding which disease processes the new therapy may be of benefit—leading to inappropriate use and less than expected results.
But, if a new treatment is considered (as with old standards of care) only when the pathology of the disease makes the use of the new therapy appropriate, results will be optimal, and we can avoid the proverbial throwing out the baby with the bathwater when the new therapy “doesn’t work” when we use the new therapy for a problem for which it would not be likely to help.
With this general idea about the relation of the mechanism of therapy and pathology of disease in mind, consider that (except for secondary results, which we will discuss in later sections) the only conditions that PRP may help are those in which strategic injection into tissue will improve the disease by improving the health of the tissue: neovascularization, neurogenesis, collagen production, improvement of glandular function, attenuation of autoimmune processes, fighting infection (all of which PRP has been documented to do).
Striated Urogenital Sphincter Grows Weaker
In considering where improved tissue health might improve stress urinary incontinence in women, first consider the striated component of the urogenital sphincter—it accounts for one-third of the resting urethral closing pressure Delancey2017
Here’s my sketch of the striated muscle component—taken from diagrams published by Delancey and others:
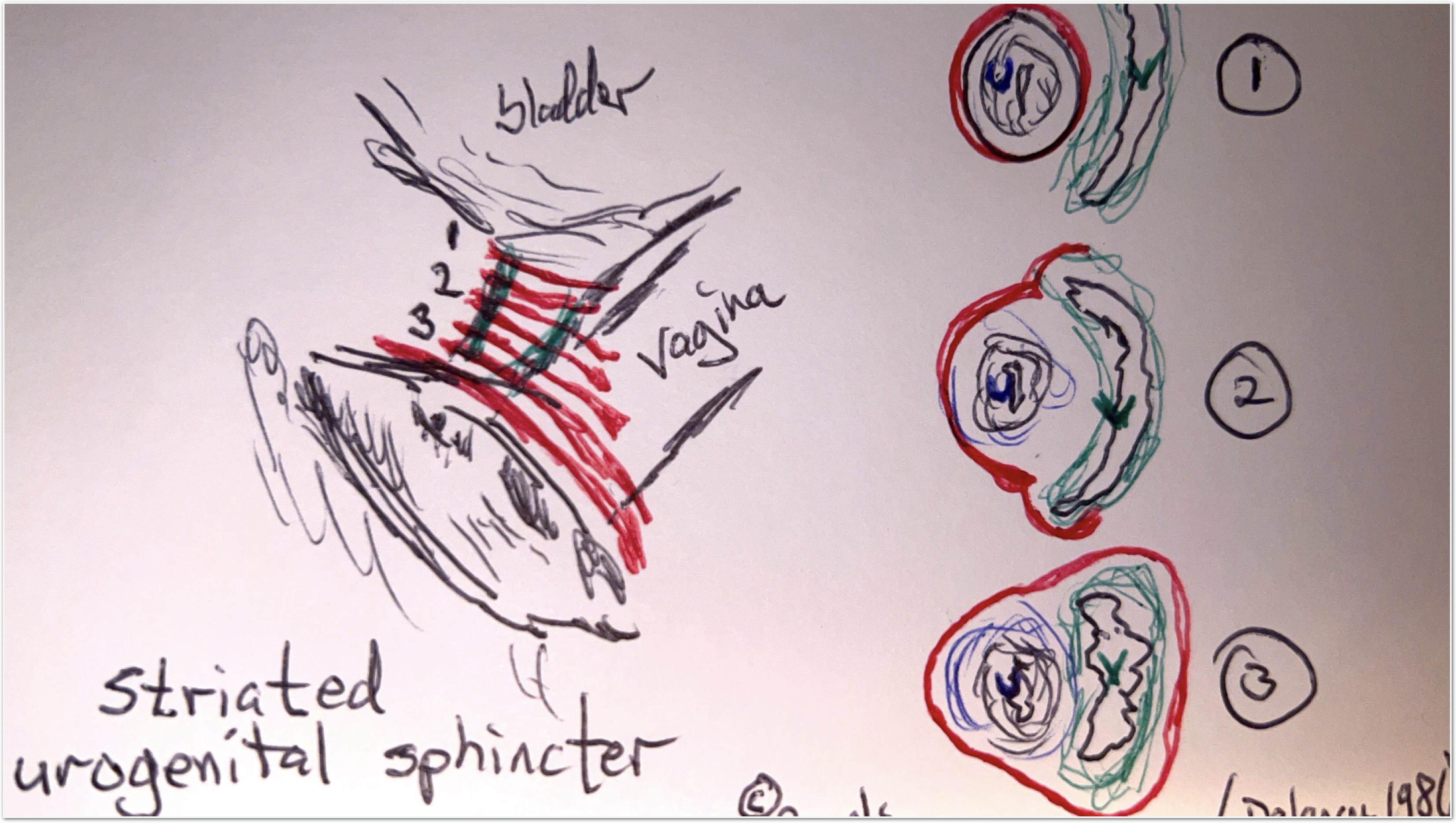
The striated urogenital sphincter completely encompasses the urethra at level one, while, at level three, it encompasses both the urethra and the vagina.
Beneath the striated urogenital sphincter lies smooth muscle that runs longitudinally, which also contributes to the closing pressure.
Just like the striated muscle of the bicep or the thigh, the number of muscle fibers in the urogenital sphincter decreases with age. Also, the number of nerves innervating the sphincter decreases with age.
To complicate matters, even more, the function of the urogenital sphincter is known to be damaged by childbirth.
Does the decrease in innervation lead to the decrease in muscle fibers, or is the decrease in muscle and the decrease in nerves two independently evolving conditions? And does blood flow play a role? I could find no clear answer to these questions. But, whatever your answer to those questions, they prompt corollary ideas such as that the effect of voluntary Kegels may be attenuated by the decreased innervation of the striated muscle (explaining the lack of effectiveness of Kegels in some women).
Treatment Strategies Based on the Functional Anatomy of the Sphincter
Activation of the striated muscle of the sphincter independently of the patient’s volition or the innervation of the muscle (for example, with an Emsella® magnet) would possibly create more contraction than would be possible even with heroic efforts by the woman. This super activation would cause a strengthening of the striated sphincter and an increase in closing pressure.
Also, the sports-medicine literature offers robust support for the idea of using PRP to restore damaged or atrophic muscle. And multiple papers demonstrate neurogenesis propagated by the injection of PRP.
If we propagated neurogenesis and muscle fiber restoration with PRP, then that might be synergistic with Kegels or with magnet therapy or with surgery (if needed).
The Urethral Wall Acts Like a Penis
The urethra wall (not the surrounding tissue, the urethra itself) carries a vascular plexus with AV anastomoses; blood flow can be directed into or away from these venues to inflate or deflate them; so it demonstrates tumescence similar to that of the penis. But in the female urethra, tumescence contributes to the closing pressure of the urethra (not erectile function, as in the man).
Hormones are known to affect this tumescence-like function of the urethra. But, what are hormones but messengers to tell cells what to do? Messages-to-the-cells is exactly what happens when the cells of the urethral wall are exposed to the small peptide chains released from platelets.
PRP was shown in a recent double-blind placebo-controlled study to improve the erectile function of the penis. Since PRP helps with neovascularization in general and has been shown to improve erectile function, it seems logical that PRP may also, when injected into the urethral wall, improve the tumescent function of the urethral wall and the closing pressure of the urethra—resulting in a decrease or resolution of urinary incontinence.
Longitudinal Smooth Muscle of the Urethra
The longitudinal smooth muscle of the urethra also contributes to the closing pressure. The smooth muscle cannot be contracted by either volition or by a magnet, so neither would help strengthen the smooth muscle component of the female urinary sphincter. But, as we have documented with the studies in our bibliography, PRP has been shown to revive muscle fibers. So, injection of the urethral smooth muscle may also account for some of the benefits of PRP seen when it is injected into the periurethral area.
And of course, such benefits of PRP may also be of help post-op from a mid-urethral sling placement. One study documented that the nerves and blood vessels between the anterior vaginal wall and the urethra are damaged by the surgical placement of a mid-urethral sling; and we have just discussed how PRP can repair nerves and blood vessels.
Urge Incontinence
Urge incontinence, multifactorial and often seen in combination with stress incontinence, can be partly secondary to peripheral nerve involvement as previously mentioned. And research supports the idea that PRP may improve the function of those nerves resulting in an improvement in urge incontinence—if the PRP should be deposited in the proper place.
PRP Injection Strategies for the Treatment of Urinary Incontinence
So, it makes sense that all of the above-mentioned ideas, if applied to the urinary sphincter in a female, might show synergistic benefits. Indeed, multiple papers do show that injection of PRP into the periurethral area or into the urethral wall improves stress urinary incontinence.
In these published papers showing benefits for stress urinary incontinence with the injection of PRP, multiple techniques have been used; so, let’s think about some of those techniques while keeping the functional anatomy in mind—looking for the best possible strategy (at least with our present knowledge).
The O-Shot® Procedure for Urinary Incontinence
With our O-Shot® procedure, we usually do two injections, one of which goes into the vaginal wall, hydro-dissecting the entire area.
Four CCs is enough to fill the whole space between the urethra and the vaginal wall and include the urethral wall—if you put the needle where it to needs to go.
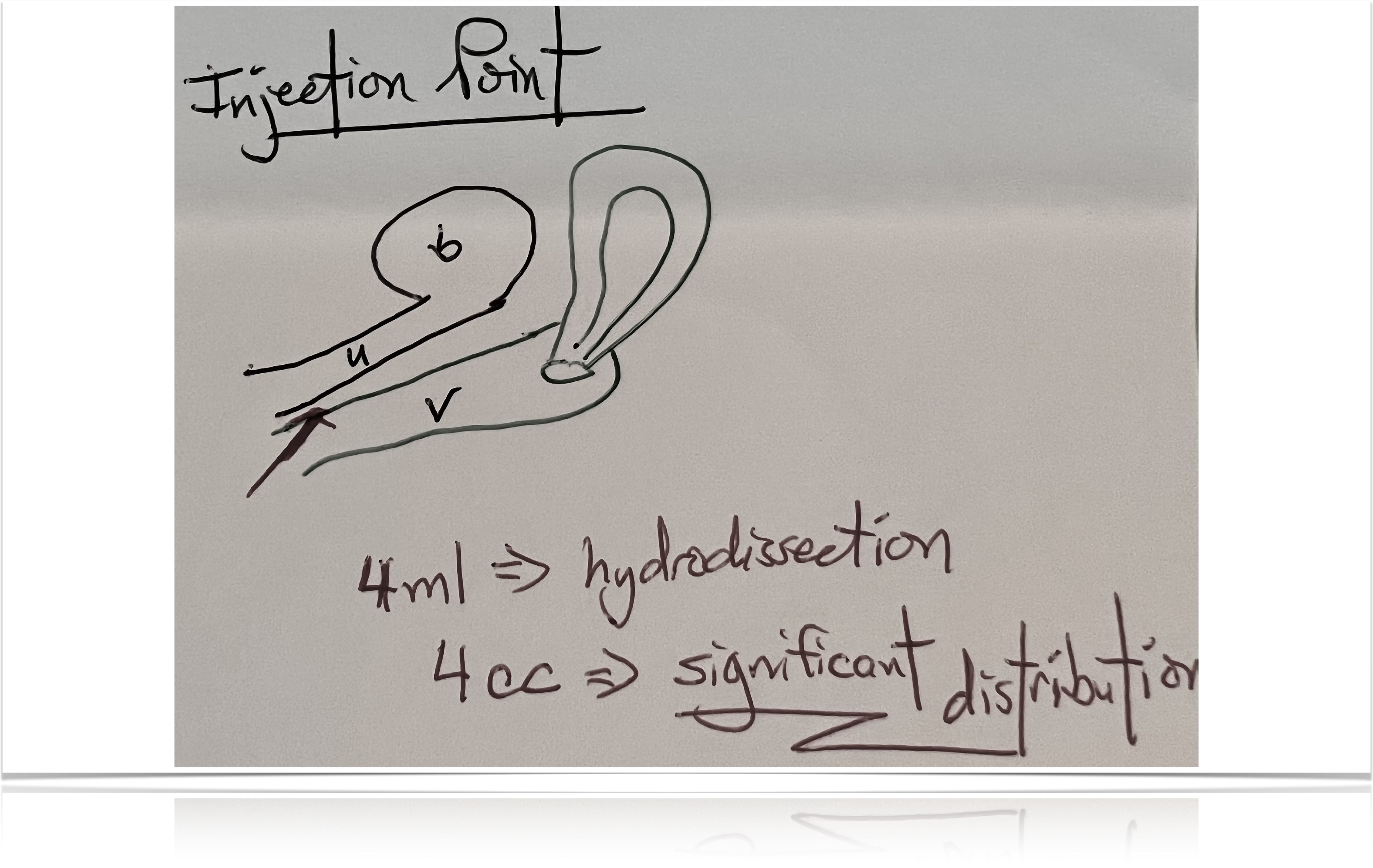
Just like with an IV, there’s variability, both in technique and with the skill of the person doing the procedure; but if you can put the needle where it belongs, you should be able to put PRP in the areas we’ve described.
The following is a snapshot from one of our instructional videos showing one of the two injections the way we teach the O-Shot® procedure for SUI. By injecting the actual anterior vaginal wall within a few millimeters of the hymenal remnant, you avoid the pain fibers within heart’s line, and you’re able to fill both the space between urethra and vagina and affect muscle, blood flow, and nerves. This can be done pain-free or near pain-free using the proper technique and only a topical cream for anesthesia.
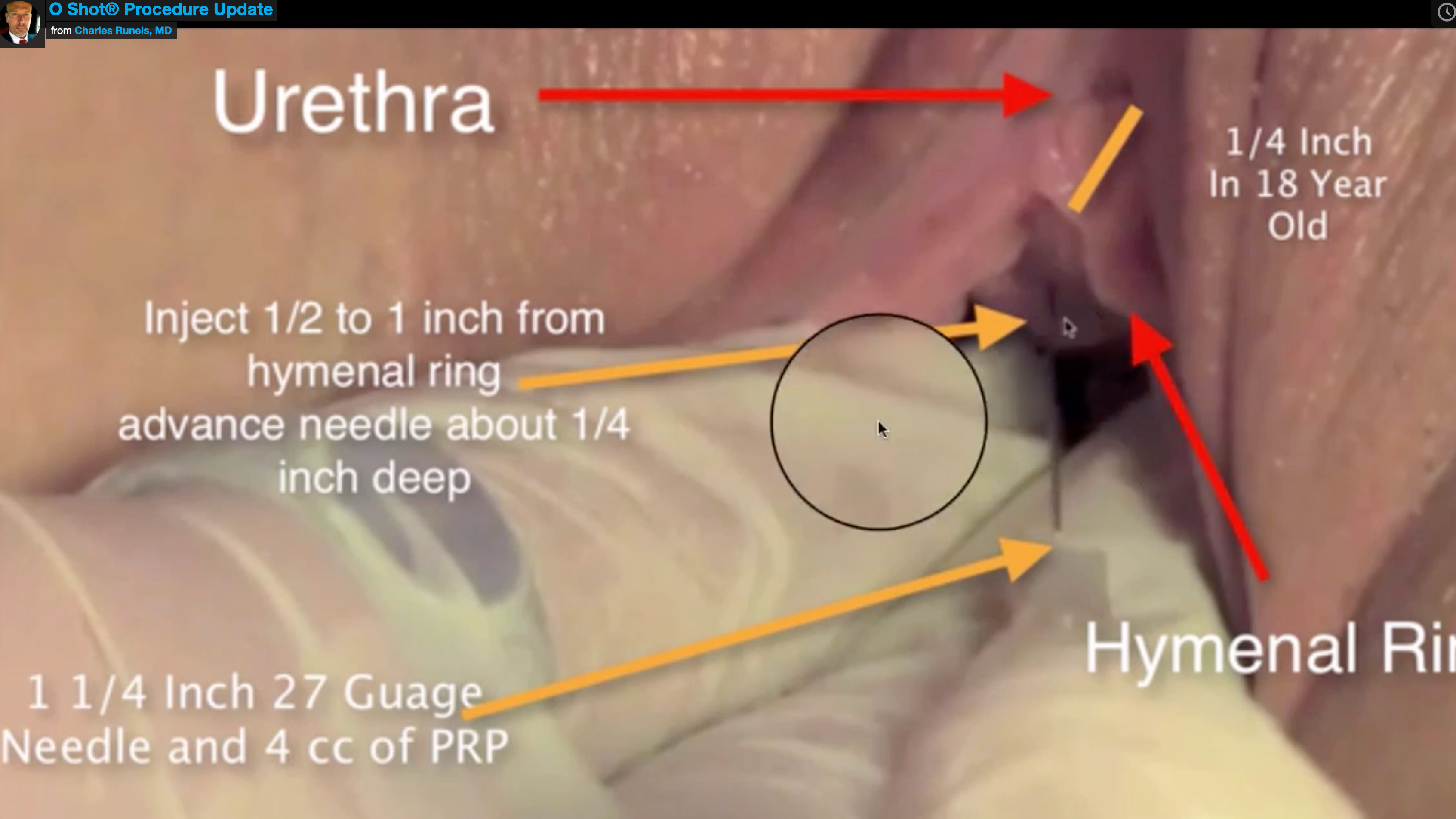
This method, when done properly, is called the O-Shot® procedure. The procedure can be done in the office using the person’s own blood and without pain in most cases.
I trademarked the term “O-Shot” to prevent variability of techniques with the associated variability of results being pushed upon women. All of the licensed providers of the O-Shot® procedure agree to follow a standard protocol with variations based on disease process. All licensed providers also agree only to use devices to prepare the PRP that has been approved by the FDA for the preparation of PRP to go back into the body. Our licensees are tested and are subject to losing the license to use the name “O-Shot®” in advertising if they fail to follow our standards.
Physicians can apply to receive more detailed instructions and to be licensed to perform the O-Shot® procedure here—>: OShot.com/physicians<—
Example of Another Technique for Curing Urinary Incontinence with PRP
Another group demonstrated (I think in a very brilliant and useful study) the resolution of urinary incontinence with the injection of PRP directly into the urethral wall—in women with objectively-demonstrated severe incontinence.
But, in their method, they report that the procedure was so painful that the subjects had to receive the urethral sphincter injection under intravenous general anesthesia in the operating room.
The following photograph illustrates the injection points:
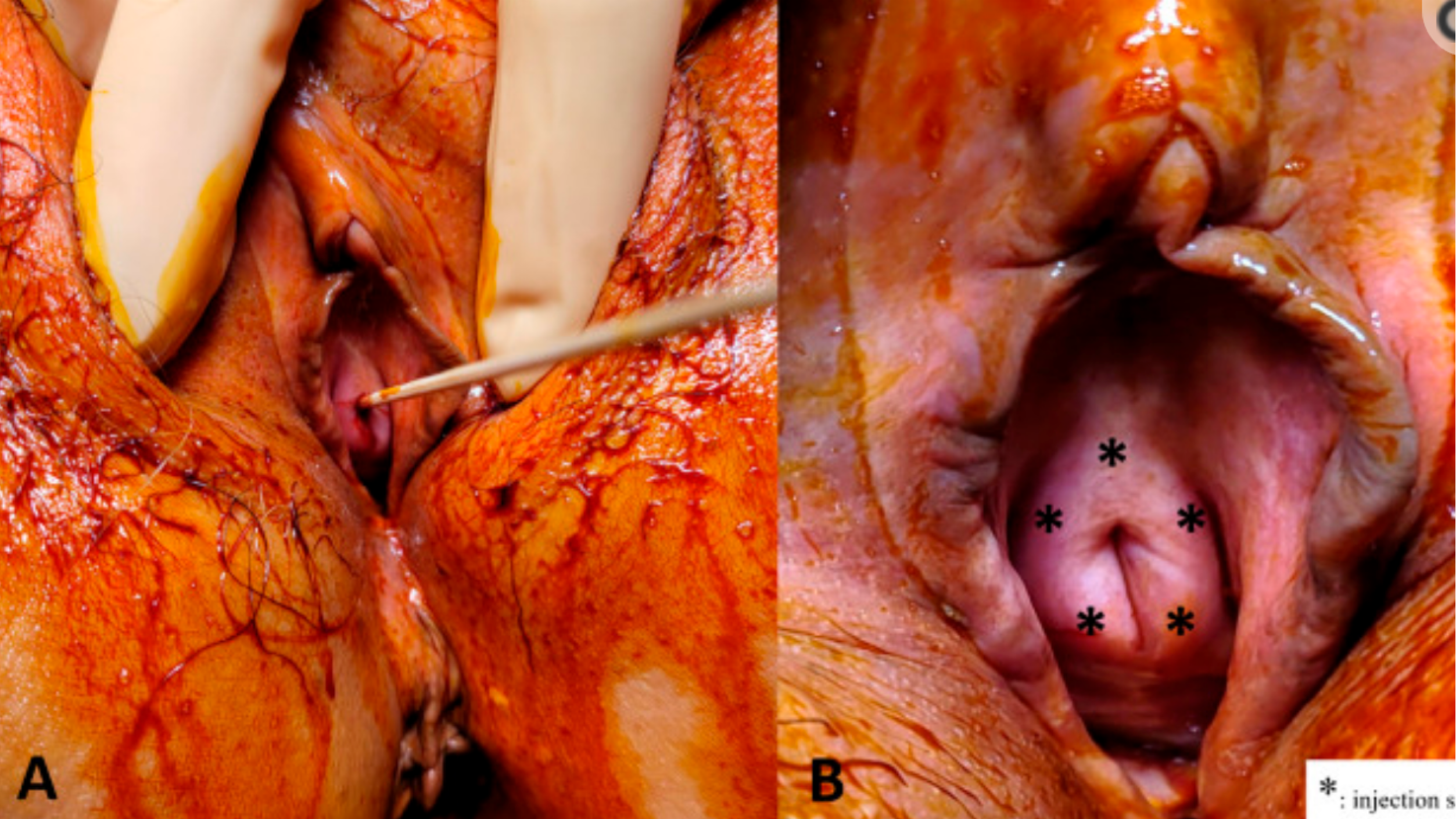
This is NOT the O-Shot® procedure; the O-Shot® procedure is a method of choosing the proper patient, properly preparing the PRP, and injecting the PRP with the agreed-upon technique after using local anesthesia that gives the best chance of an in-office, pain-free procedure.
But, though these investigators did something other than an O-Shot® procedure, they did help the mission of finding a way to cure or improve female urinary incontinence by demonstrating the possibility of improving the health and function of the urinary sphincter by using a functional-anatomy-based, strategic injection of PRP.
Another third technique (not pictured here) showed benefit for SUI but described injecting 4 cc of PRP spread out in 0.1 ml aliquots for FORTY separate injection points.
Please Help
I have no study showing which of the three separate techniques described above gives the best result. There is no question about which causes the least amount of pain: (1) 40 separate injections vs. (2) five injections into the urethral wall requiring general anesthesia in the operating room vs. (3) the O-Shot® which can be done usually completely pain-free and only requires topical anesthesia and a pain-free lidocaine block in the office.
Though we still do not have a study documenting which works the best of these three techniques (or which of other techniques that you might imagine), hopefully, I have given you a quick version of why I think our method (which we have been doing for a decade with over 100,000 women treated) may be best.
More importantly, what I hope I’ve shown is that there is a need for us to think about carefully and study which might be the best technique because we think technique matters. One of the dangers of having taught and provided the O-Shot® procedure for 11 years is that I may start to believe everything I say…first you show something is feasible, then you have the herculean effort of looking at the infinite number of variables to find the best way.
Please help us study and think about this categorically new way to improve the health and function of the female genitourinary tract.
Summary
- PRP improves tissue health by collagen production, neovascularization, neurogenesis, attenuation of the autoimmune response, anti-bacterial effects, improving glandular function, and muscle repair.
- The strategic injection of PRP into areas of damaged tissue with the resultant improvement of health and function of tissue has been demonstrated in thousands of studies over the past two decades.
- An increasing number of studies show that this principle of injection of PRP into the damaged or aging tissue of the urinary sphincter may help restore urinary continence in some women resistant to other therapies.
- The injection technique matters since only tissue exposed to the PRP will primarily benefit.
- Only those licensed by the Cellular Medicine Association (after testing) can legally advertise using the name “O-Shot®.” This is done so that a greater degree of predictability of safety and results can be offered to women who may choose PRP injections as a mode of treatment.
- The O-Shot® procedure has and will continue to evolve as the research accumulates from the members of the Cellular Medicine Association and others.
- The O-Shot® procedure is varied based on the functional anatomy and the pathophysiology of the disease process in any individual woman.
- This report gives an overview of the principles behind the O-Shot® procedure and Emsella therapy but does not constitute training or license to do the procedures.
- I hope if you are not yet a member of the Cellular Medicine Association (CMA) that you will consider joining our organization and studying our training materials, we need more help thinking about and researching these ideas:
- If you are already a member of the CMA, I hope you will continue to support our mission by participating in our weekly journal club and sharing your observations with your patients and your thoughts about the current research.
My goal is to be a pipe for the movement of ideas. I continue to be grateful every day for the members of the CMA who have shared ideas that make this report possible. And, most of all, I am grateful for the women who have been patients who have trusted me to teach me; with old ideas and new, the best book is observing and listening to the one woman in front of you who will teach you about her disease and how to make her well—if you listen.
References
Selection of Papers Demonstrating Neurogenesis with PRP
Chung, Eric. “Regenerative Technology to Restore and Preserve Erectile Function in Men Following Prostate Cancer Treatment: Evidence for Penile Rehabilitation in the Context of Prostate Cancer Survivorship.” Therapeutic Advances in Urology 13 (January 1, 2021): 17562872211026420. https://doi.org/10.1177/17562872211026421.
Foy, Christian A., William F. Micheo, and Damien P. Kuffler. “Functional Recovery Following Repair of Long Nerve Gaps in Senior Patient 2.6 Years Posttrauma.” Plastic and Reconstructive Surgery. Global Open 9, no. 9 (September 2021): e3831. https://doi.org/10.1097/GOX.0000000000003831.
Kuffler, Damien P. “Platelet-Rich Plasma and the Elimination of Neuropathic Pain.” Molecular Neurobiology 48, no. 2 (October 2013): 315–32. https://doi.org/10.1007/s12035-013-8494-7.
Sánchez, Mikel, Eduardo Anitua, Diego Delgado, Peio Sanchez, Roberto Prado, Gorka Orive, and Sabino Padilla. “Platelet-Rich Plasma, a Source of Autologous Growth Factors and Biomimetic Scaffold for Peripheral Nerve Regeneration.” Expert Opinion on Biological Therapy 17, no. 2 (February 1, 2017): 197–212. https://doi.org/10.1080/14712598.2017.1259409.
Wu, Yi-No, Chun-Hou Liao, Kuo-Chiang Chen, and Han-Sun Chiang. “Dual Effect of Chitosan Activated Platelet Rich Plasma (CPRP) Improved Erectile Function after Cavernous Nerve Injury.” Journal of the Formosan Medical Association, March 27, 2021. https://doi.org/10.1016/j.jfma.2021.01.019.
Selection of Papers Demonstrating Muscle Revival from PRP
Bernuzzi, Gino, Federica Petraglia, Martina Francesca Pedrini, Massimo De Filippo, Francesco Pogliacomi, Michele Arcangelo Verdano, and Cosimo Costantino. “Use of Platelet-Rich Plasma in the Care of Sports Injuries: Our Experience with Ultrasound-Guided Injection.” Blood Transfusion 12, no. Suppl 1 (January 2014): s229–34. https://doi.org/10.2450/2013.0293-12.
Bubnov, Rostyslav, Viacheslav Yevseenko, and Igor Semeniv. “Ultrasound Guided Injections of Platelets Rich Plasma for Muscle Injury in Professional Athletes. Comparative Study.,” n.d., 5.
Le, Adrian D.K., Lawrence Enweze, Malcolm R. DeBaun, and Jason L. Dragoo. “Platelet-Rich Plasma.” Clinics in Sports Medicine 38, no. 1 (January 2019): 17–44. https://doi.org/10.1016/j.csm.2018.08.001.
Middleton, Kellie K, Victor Barro, Bart Muller, Satosha Terada, and Freddie H Fu. “Evaluation of the Effects of Platelet-Rich Plasma (PRP) Therapy Involved in the Healing of Sports-Related Soft Tissue Injuries.” The Iowa Orthopaedic Journal 32 (2012): 150–63. http://www.ncbi.nlm.nih.gov/pubmed/23576936.
Moraes, Vinícius Y, Mário Lenza, Marcel Jun Tamaoki, Flávio Faloppa, and João Carlos Belloti. “Platelet-Rich Therapies for Musculoskeletal Soft Tissue Injuries.” The Cochrane Database of Systematic Reviews 12 (January 2013): CD010071. https://doi.org/10.1002/14651858.CD010071.pub2.
Selection of Papers Showing Help from PRP Injections for Stress Urinary Incontinence
Athanasiou, Stavros, Christos Kalantzis, Dimitrios Zacharakis, Nikolaos Kathopoulis, Artemis Pontikaki, and Themistoklis Grigoriadis. “The Use of Platelet-Rich Plasma as a Novel Nonsurgical Treatment of the Female Stress Urinary Incontinence: A Prospective Pilot Study.” Female Pelvic Medicine & Reconstructive Surgery 27, no. 11 (November 2021): e668–72. https://doi.org/10.1097/SPV.0000000000001100.
Callewaert, Geertje, Marina Monteiro Carvalho Mori Da Cunha, Nikhil Sindhwani, Maurilio Sampaolesi, Maarten Albersen, and Jan Deprest. “Cell-Based Secondary Prevention of Childbirth-Induced Pelvic Floor Trauma.” Nature Reviews Urology 14, no. 6 (June 2017): 373–85. https://doi.org/10.1038/nrurol.2017.42.
Indian Journal of Medical Ethics. “Cosmetic Surgical Procedures on the Vulva and Vagina – an Overview.” Accessed January 18, 2022. https://ijme.in/articles/cosmetic-surgical-procedures-on-the-vulva-and-vagina-an-overview/.
Ford, Abigail A., Lynne Rogerson, June D. Cody, and Joseph Ogah. “Mid‐urethral Sling Operations for Stress Urinary Incontinence in Women.” Cochrane Database of Systematic Reviews, no. 7 (2015). https://doi.org/10.1002/14651858.CD006375.pub3.
Gorton, E, S Stanton, A Monga, A K Wiskind, G M Lentz, and D R Bland. “Periurethral Collagen Injection: A Long-Term Follow-up Study.” BJU International 84, no. 9 (December 1999): 966–71. http://www.ncbi.nlm.nih.gov/pubmed/10571621.
Joseph, Christine, Kosha Srivastava, Olive Ochuba, Sheila W. Ruo, Tasnim Alkayyali, Jasmine K. Sandhu, Ahsan Waqar, Ashish Jain, and Sujan Poudel. “Stress Urinary Incontinence Among Young Nulliparous Female Athletes.” Cureus 13, no. 9 (September 2021). https://doi.org/10.7759/cureus.17986.
Kirchin, Vivienne, Tobias Page, Phil E. Keegan, Kofi OM Atiemo, June D. Cody, Samuel McClinton, Patricia Aluko, and Cochrane Incontinence Group. “Urethral Injection Therapy for Urinary Incontinence in Women.” The Cochrane Database of Systematic Reviews 2017, no. 7 (July 2017). https://doi.org/10.1002/14651858.CD003881.pub4.
Lee, Patricia E., Rose C. Kung, and Harold P. Drutz. “PERIURETHRAL AUTOLOGOUS FAT INJECTION AS TREATMENT FOR FEMALE STRESS URINARY INCONTINENCE: A RANDOMIZED DOUBLE-BLIND CONTROLLED TRIAL.” Journal of Urology 165, no. 1 (January 2001): 153–58. https://doi.org/10.1097/00005392-200101000-00037.
Long, Cheng-Yu, Kun-Ling Lin, Chin-Ru Shen, Chin-Ru Ker, Yi-Yin Liu, Zi-Xi Loo, Hui-Hua Hsiao, and Yung-Chin Lee. “A Pilot Study: Effectiveness of Local Injection of Autologous Platelet-Rich Plasma in Treating Women with Stress Urinary Incontinence.” Scientific Reports 11, no. 1 (December 2021): 1584. https://doi.org/10.1038/s41598-020-80598-2.
Nikolopoulos, Kostis I., Vasilios Pergialiotis, Despina Perrea, and Stergios K. Doumouchtsis. “Restoration of the Pubourethral Ligament with Platelet Rich Plasma for the Treatment of Stress Urinary Incontinence.” Medical Hypotheses 90 (May 2016): 29–31. https://doi.org/10.1016/j.mehy.2016.02.019.
O’Connor, Eabhann, Aisling Nic an Riogh, Markos Karavitakis, Serenella Monagas, and Arjun Nambiar. “Diagnosis and Non-Surgical Management of Urinary Incontinence – A Literature Review with Recommendations for Practice.” International Journal of General Medicine 14 (August 16, 2021): 4555–65. https://doi.org/10.2147/IJGM.S289314.
Oshiro, Takuma, Ryu Kimura, Keiichiro Izumi, Asuka Ashikari, Seiichi Saito, and Minoru Miyazato. “Changes in Urethral Smooth Muscle and External Urethral Sphincter Function with Age in Rats.” Physiological Reports 8, no. 24 (2021): e14643. https://doi.org/10.14814/phy2.14643.
PANDIT, MEGHANA, JOHN O. L. DELANCEY, JAMES A. ASHTON-MILLER, JYOTHSNA IYENGAR, MILA BLAIVAS, and DANIELE PERUCCHINI. “Quantification of Intramuscular Nerves Within the Female Striated Urogenital Sphincter Muscle.” Obstetrics and Gynecology 95, no. 6 Pt 1 (June 2000): 797–800. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1192577/.
Perucchini, Daniele, John O.L. DeLancey, James A. Ashton-Miller, Andrzej Galecki, and Gabriel N. Schaer. “Age Effects on Urethral Striated Muscle II. Anatomic Location of Muscle Loss.” American Journal of Obstetrics and Gynecology 186, no. 3 (March 2002): 356–60. https://doi.org/10.1067/mob.2002.121090.
Perucchini, Daniele, John OL DeLancey, James A. Ashton-Miller, Ursula Peschers, and Tripti Kataria. “Age Effects on Urethral Striated Muscle I. Changes in Number and Diameter of Striated Muscle Fibers in the Ventral Urethra.” American Journal of Obstetrics & Gynecology 186, no. 3 (March 1, 2002): 351–55. https://doi.org/10.1067/mob.2002.121089.
Wiśniewska-Ślepaczuk, Katarzyna, Agnieszka Pieczykolan, Joanna Grzesik-Gąsior, and Artur Wdowiak. “A Review of Aesthetic Gynecologic Procedures for Women.” Plastic Surgical Nursing 41, no. 4 (October 2021): 191–202. https://doi.org/10.1097/PSN.0000000000000400.
Zhou, Shukui, Kaile Zhang, Anthony Atala, Oula Khoury, Sean V Murphy, Weixin Zhao, and Qiang Fu. “Stem Cell Therapy for Treatment of Stress Urinary Incontinence: The Current Status and Challenges,” n.d. https://doi.org/10.1155/2016/7060975.
Zubieta, Maria, Rebecca L. Carr, Marcus J. Drake, and Kari Bø. “Influence of Voluntary Pelvic Floor Muscle Contraction and Pelvic Floor Muscle Training on Urethral Closure Pressures: A Systematic Literature Review.” International Urogynecology Journal 27, no. 5 (May 2016): 687–96. https://doi.org/10.1007/s00192-015-2856-9.
Lee, Ping-Jui, Yuan-Hong Jiang, and Hann-Chorng Kuo. “A Novel Management for Postprostatectomy Urinary Incontinence: Platelet-Rich Plasma Urethral Sphincter Injection.” Scientific Reports | 11 (123AD): 5371. https://doi.org/10.1038/s41598-021-84923-1.
Chiang, Ching-Hsiang, and Hann-Chorng Kuo. “The Efficacy and Mid-Term Durability of Urethral Sphincter Injections of Platelet-Rich Plasma in Treatment of Female Stress Urinary Incontinence.” Frontiers in Pharmacology 13 (February 8, 2022): 847520. https://doi.org/10.3389/fphar.2022.847520.
Selection of Papers Demonstrating Improvement of SUI with Magnet (Emsella®)
Azparren, Javier, and Judson Brandeis. “HIFEM PROCEDURE ENHANCES QUALITY OF LIFE OF ELDERLY MEN WITH POST-PROSTATECTOMY INCONTINENCE,” n.d., 6.
Evans, Kimberly, and Julene B Samuels. “FEMALE URINARY INCONTINENCE AND SEXUAL FUNCTION AFTER THE HIFEM® PROCEDURE,” n.d., 2.
Gözlersüzer, Özlem, Bestami Yalvaç, and Basri Çakıroğlu. “Investigation of the Effectiveness of Magnetic Field Therapy in Women with Urinary Incontinence: Literature Review.” Urologia Journal, January 9, 2022, 03915603211069010. https://doi.org/10.1177/03915603211069010.
He, Qing, Kaiwen Xiao, Liao Peng, Junyu Lai, Hong Li, Deyi Luo, and Kunjie Wang. “An Effective Meta-Analysis of Magnetic Stimulation Therapy for Urinary Incontinence.” Scientific Reports 9 (June 24, 2019): 9077. https://doi.org/10.1038/s41598-019-45330-9.
Samuels, Julene B. “HIFEM TECHNOLOGY – THE NON-INVASIVE TREATMENT OF URINARY INCONTINENCE,” n.d., 7.
Samuels, Julene B., Andrea Pezzella, Joseph Berenholz, and Red Alinsod. “Safety and Efficacy of a Non‐Invasive High‐Intensity Focused Electromagnetic Field (HIFEM) Device for Treatment of Urinary Incontinence and Enhancement of Quality of Life.” Lasers in Surgery and Medicine 51, no. 9 (November 2019): 760–66. https://doi.org/10.1002/lsm.23106.
Silantyeva, Elena, Dragana Zarkovic, Evgeniia Astafeva, Ramina Soldatskaia, Mekan Orazov, Marina Belkovskaya, Mark Kurtser, and Academician of the Russian Academy of Sciences. “A Comparative Study on the Effects of High-Intensity Focused Electromagnetic Technology and Electrostimulation for the Treatment of Pelvic Floor Muscles and Urinary Incontinence in Parous Women: Analysis of Posttreatment Data.” Female Pelvic Medicine & Reconstructive Surgery 27, no. 4 (April 2021): 269–73. https://doi.org/10.1097/SPV.0000000000000807.
Another Selection of Papers Showing Neovacularization from PRP
Araujo-Gutierrez, Raquel, Jeffrey L. Van Eps, Jacob C. Scherba, Albert Thomas Anastasio, Fernando Cabrera, Cory J. Vatsaas, Keith Youker, and Joseph S. Fernandez Moure. “Platelet Rich Plasma Concentration Improves Biologic Mesh Incorporation and Decreases Multinucleated Giant Cells in a Dose Dependent Fashion.” Journal of Tissue Engineering and Regenerative Medicine 15, no. 11 (2021): 1037–46. https://doi.org/10.1002/term.3247.
Bindal, Priyadarshni, Nareshwaran Gnanasegaran, Umesh Bindal, Nazmul Haque, Thamil Selvee Ramasamy, Wen Lin Chai, and Noor Hayaty Abu Kasim. “Angiogenic Effect of Platelet-Rich Concentrates on Dental Pulp Stem Cells in Inflamed Microenvironment.” Clinical Oral Investigations 23, no. 10 (October 2019): 3821–31. https://doi.org/10.1007/s00784-019-02811-5.
Li, Yuan, Shan Mou, Peng Xiao, Guining Li, Jialun Li, Jing Tong, Jiecong Wang, Jie Yang, Jiaming Sun, and Zhenxing Wang. “Delayed Two Steps PRP Injection Strategy for the Improvement of Fat Graft Survival with Superior Angiogenesis.” Scientific Reports 10 (March 23, 2020): 5231. https://doi.org/10.1038/s41598-020-61891-6.
Nolan, Grant Switzer, Oliver John Smith, Susan Heavey, Gavin Jell, and Afshin Mosahebi. “Histological Analysis of Fat Grafting with Platelet‐rich Plasma for Diabetic Foot Ulcers—A Randomised Controlled Trial.” International Wound Journal 19, no. 2 (June 24, 2021): 389–98. https://doi.org/10.1111/iwj.13640.
Norooznezhad, Amir Hossein. “Decreased Pain in Patients Undergoing Pilonidal Sinus Surgery Treated with Platelet-Rich Plasma Therapy: The Role of Angiogenesis.” Advances in Skin & Wound Care 33, no. 1 (January 2020): 8. https://doi.org/10.1097/01.ASW.0000615376.97232.0a.
Saputro, Iswinarno Doso, Sitti Rizaliyana, and Dhitta Aliefia Noverta. “The Effect of Allogenic Freeze-Dried Platelet-Rich Plasma in Increasing the Number of Fibroblasts and Neovascularization in Wound Healing.” Annals of Medicine and Surgery 73 (January 3, 2022): 103217. https://doi.org/10.1016/j.amsu.2021.103217.
Sclafani, Anthony P., and Steven A. McCormick. “Induction of Dermal Collagenesis, Angiogenesis, and Adipogenesis in Human Skin by Injection of Platelet-Rich Fibrin Matrix.” Archives of Facial Plastic Surgery 14, no. 2 (April 2012): 132–36. https://doi.org/10.1001/archfacial.2011.784.
Zhang, X.-L., K.-Q. Shi, P.-T. Jia, L.-H. Jiang, Y.-H. Liu, X. Chen, Z.-Y. Zhou, Y.-X. Li, and L.-S. Wang. “Effects of Platelet-Rich Plasma on Angiogenesis and Osteogenesis-Associated Factors in Rabbits with Avascular Necrosis of the Femoral Head.” European Review for Medical and Pharmacological Sciences 22, no. 7 (April 2018): 2143–52. https://doi.org/10.26355/eurrev20180414748.
An Optional Technique for Injecting PRP for Incontinence
Chiang, Ching-Hsiang, and Hann-Chorng Kuo. “The Efficacy and Mid-Term Durability of Urethral Sphincter Injections of Platelet-Rich Plasma in Treatment of Female Stress Urinary Incontinence.” Frontiers in Pharmacology 13 (February 8, 2022): 847520. https://doi.org/10.3389/fphar.2022.847520
The Effects of Mid-Urethral Sling Placement on the Tissue of the Female Prostate and Female Sexual Function
Gaudet, D., D.G. Clohosey, J.L. Hannan, S.W. Goldstein, N. Szell, B.R. Komisarek, M.A. Harvey, et al. “249 Midurethral Sling Placement Disrupts Periurethral Neurovascular and Glandular Structures near Anterior Vaginal Wall: Potential Role in Female Sexual Dysfunction.” The Journal of Sexual Medicine 15, no. 7 (July 2018): S221–22. https://doi.org/10.1016/j.jsxm.2018.04.214.






Leave a Reply